What Is the Shape of an P Orbital
In the figure above the dashed line is the focus for why the p orbital has a different shape than the s orbital. The maximum number of electrons that can be placed in an p orbital.
It is not likely because the largest part of the orbitals shape peanut-d clover-d is away from the nucleus.

. View solution Assertion p-orbital is dumb-bell shaped. If you describe an electron distribution with one of these functions you can plot contours at a chosen value and these contours have shapes. A p orbital is a dumbbell shape or lobed region describing where an electron can be found with a certain degree of probability.
The p orbital has six protons to complete the third level of a tetrahedral structure. A p orbital has the approximate shape of a pair of lobes on opposite sides of the nucleus or a somewhat dumbbell shape. 5 What does size of orbital depend on.
Diagram of the S and P orbitals. This orbital fill after the s the shape is a dumbbell. Maximum number of electrons that can be placed in an s orbital.
11 Which quantum number determines the shape orientation. It means that p orbitals can have three possible orientations. For p-subshell l 1 there are three values of m namely -1 0 1.
The shape of the orbital if azimuthal quantum number is unity is. The p orbital is dumbbell shaped in which two lobes are emerging out from a common centre in opposite directions. Shapes and Energies of Atomic Orbitals Draw the structure of p - orbitals.
The p orbital appears as a dumbbell a spherical shape like the s orbital cut in half. How likely it is that an electron occupaying a p or a d in an orbital would be found very near an atoms nucleus. Each sphere is a single orbital.
The orbital of p behaves as a dumbbell-a circular shape split in half like the orbital of s. Each p-orbital consists of two lobes symmetrical about a particular axis. Specific protons also rotate like the atomic nucleus spins.
A p orbital is a 3D-function and these functions dont have shapes they have values at any point in space. The shape of the orbital depends on the quantum numbers associated with an energy state. P subshells are made up of three dumbbell-shaped orbitals.
6 What is orbit in geography. Draw the structure of p-orbitals. Shape of p-orbitals.
The shape of the p orbital is dumb-Bell. The direction characteristics of p orbital enable it. Depending upon the orientation of the lobes these are denoted as 2p x 2p y and 2p z accordingly as they are symmetrical about X Y and Z axis respectively.
Spherical orbital also the lowest energy orbital. 90 and 270. 5 What is the shape of the fixed orbit.
The maximum number of electrons that can be placed in an d orbital. Lets have a closer look at the. Both the 1n and 2n principal shells have an s orbital but the size of the sphere is larger in the 2n orbital.
The lines in the figure represents the cross-section of the three-dimensional boundary surface of p-orbitals. What is the shape of an s orbital. 7 What is the size of the orbit.
Each of the four sub-levels has a particular form or field where it is possible to locate electrons. What are the shapes of the d orbitals. Secondly why is the s orbital spherical.
2 What is an orbit and what shape is it. Atomic orbitals describe the most likely location of the electrons that will be found around the nucleus of an atom. 8 When L 3 What values can m have.
Overview of Shape Of P Orbital According to quantum mechanics the electrons have both wave as well as particle nature. It gives rise to a dumb-bell shape of p orbital. The s subshells are shaped like spheres.
The shape of the orbital depends on the quantum numbers associated with an energy state. 3 What is the shape of orbit in an atom. Orbitals in quantum chemistry have shapes that are spheres for s-orbitals dumbbells for p-orbitals and different types of d-orbital are either pairs of crossed dumbbells or a dumbbell with a central collar.
6 Which quantum number identifies the size and energy of orbital. The node of the dumbbell occurs at the a tomic nucleus so the probability of finding an electron in the nucleus is very low but not zero. 10 What is Hunds rule in chemistry.
7 Why are orbitals different shapes. P orbital has 3 orientations. During a rotation three protons coordinate two times.
These three p-orbitals are equal in energy degenerate state but differ in their orientations. The atomic orbitals are of different shapes where the s orbital has spherical shape the p orbital has a dumbbell shape and the d orbital has a double dumbbell shape. 10 Which of the following is the distinct shape.
Of the four s and p orbitals are considered because these orbitals are the most common in organic and biological chemistry. The d orbital is a clover shape because the electron is pushed out four times during the rotation when an opposite spin proton aligns gluons with three spin-aligned protons. As the atomic nucleus spins individual protons also spin.
An s-orbital is spherical with the nucleus at its centre a p-orbitals is dumbbell-shaped and four of the five d orbitals are cloverleaf shaped. 9 What is the shape of ap orbital. 4 What determines orbital shape.
8 How are orbital shapes determined. The p orbital is a dumbbell-shaped or lobed region describing where an electron can be found within a certain degree of probability. F-orbitals have yet more complex shapes but.
There are four different kinds of orbitals denoted s p d and f each with a different shape. A d sublevel can hold a maximum of 10 electrons. The p orbital is a dumbbell-shaped or lobed region describing where an electron can be found within a certain degree of probability.
You just studied 6 terms. In this regard what shape is the s and p orbitals. Solve any question of Structure of Atom with-Patterns of problems Was this answer helpful.
Principal shell 2n has a p subshell but shell 1 does not. Orbitals define regions in space where you are likely to find electronss orbitals ℓ 0 are spherical shapedp orbitals ℓ 1 are dumb-bell shapedThe three possible p orbitals are always perpendicular to each other. All s orbitals are spherical in shape and have spherical symmetry.
Every unique orbital can comprise only upto two electrons. Each d orbital can hold a maximum of two electrons. 4 What are the 4 shapes of orbitals.
9 What determines the energy of an electron. Each p-orbital consists of two lobes symmetrical about a particular axis. What part of the diagram supports your conclusion.
As l1 the p orbital has three different orientations known as Px Py and Pz which depends on where the electron density is the maximum such as the x-axis y-axis and z-axis.

Orbital Hybridization Cheat Sheet Example Notas De Quimica Ensenanza De Quimica Geometria Molecular

Chemistry The Central Science Chapter 9 Section 5 Teaching Chemistry Chemistry Lessons Organic Chemistry

Orbital Configurations The P Orbital Is The Dumbbell Shape Going From An Extended Nucleus Like S Energy State To An Extended Higher Energy Dumbbell Like S

Chemistry Atomic Orbital Shapes Ggca Science Lab Science Lab Shapes Atomic Structure

Classifying Calm The Chaos Periodic Chart Make It Yourself Electrons

Energy Levels Sublevels And Orbitals Youtube Energy Level Energy Chemistry

Orbital Hybridization Chemistry Science Chemistry Nomenclature Chemistry

Quantum Number Orbital Diagram Priyamstudycentre Chemistry Lessons College Chemistry Chemistry

Shapes Of Hybrid Orbitals Note The Hybridisation Not Only Explains Observed Shapes But It Also Explains The Chemistry Chemistry Education Interactive Science

Electron Configuration Electron Configuration Teaching Chemistry Chemistry Lessons

Quantum Number Orbital Definition Formula Diagram Shape Chemistry Organic Chemistry Chemistry Classroom

Pin By Carlos On Chemistry Chemistry Classroom Science Chemistry Organic Chemistry

Orbital Diagram Of Carbon Teaching Chemistry Chemistry Lessons Science Chemistry

Cartoons Shapes Visualisation Cartoon
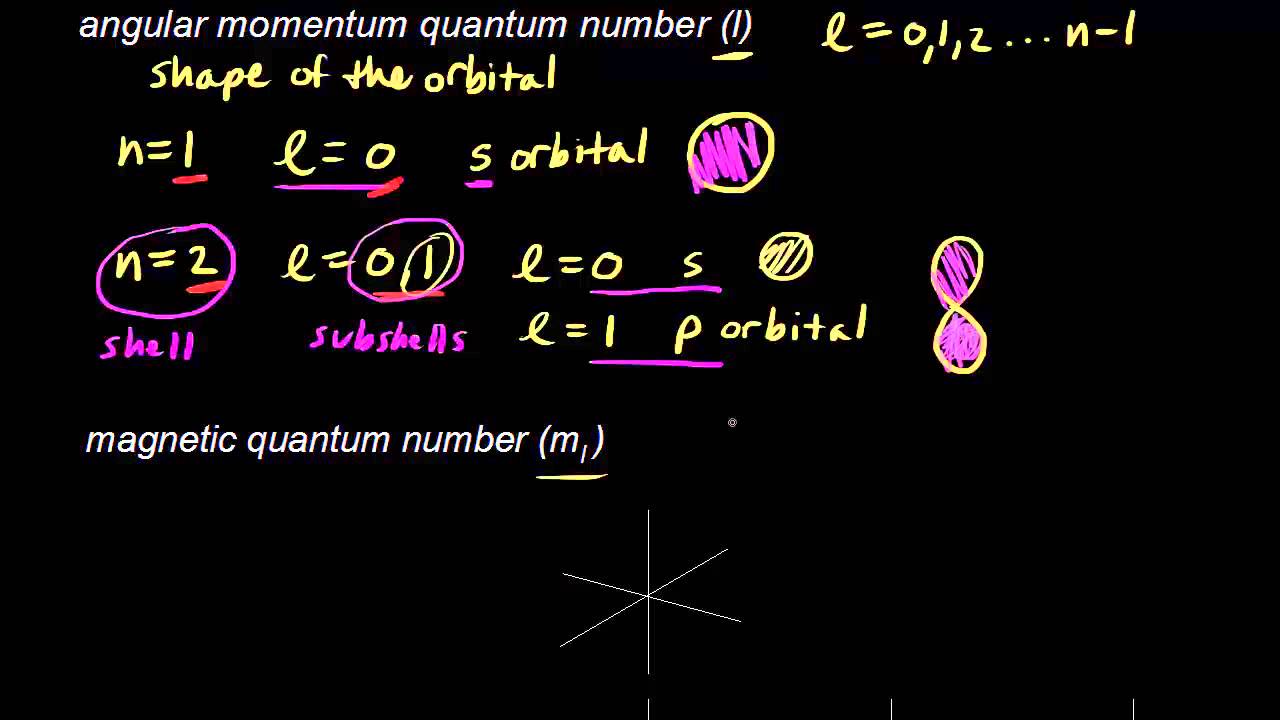
Quantum Numbers Electronic Structure Of Atoms Chemistry Khan Academy Atomic Structure Chemistry Quantum Mechanics

Chemistry Land Chemistry Education Teaching Chemistry Electron Configuration

Hybrid Orbitals And Their Corresponding Vsepr Geometries 28 Images Chapter 12 Physical Chemistry Th Teaching Chemistry Molecular Geometry Chemistry Basics



Comments
Post a Comment