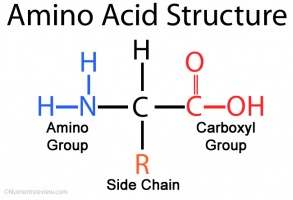
Identify the Amino Group Present in the Given Amino Acid.
View the full answer. Essential amino acids must be acquired through diet.

Amino Acids An Overview Sciencedirect Topics
Identify the amino group present in the given amino acid.
. On the given structure CH3 is present on the side chain and here the amino acid is alanine and in the second image hydrogen is present as the side chain and so it is the simplest amino acid that is glycine. The following are the tests carried to identify the presence of amino group present in the organic compound. A carbohydrate contains hydroxyl groups and several other functional groups depending on the structure.
The number of pK a values differentiates polar and nonpolar amino acids from charged amino acids. Click to see full answer. Amino acids are bifunctional organic compounds hence it contains both carboxylic group C O O H as well as amino group N H 2.
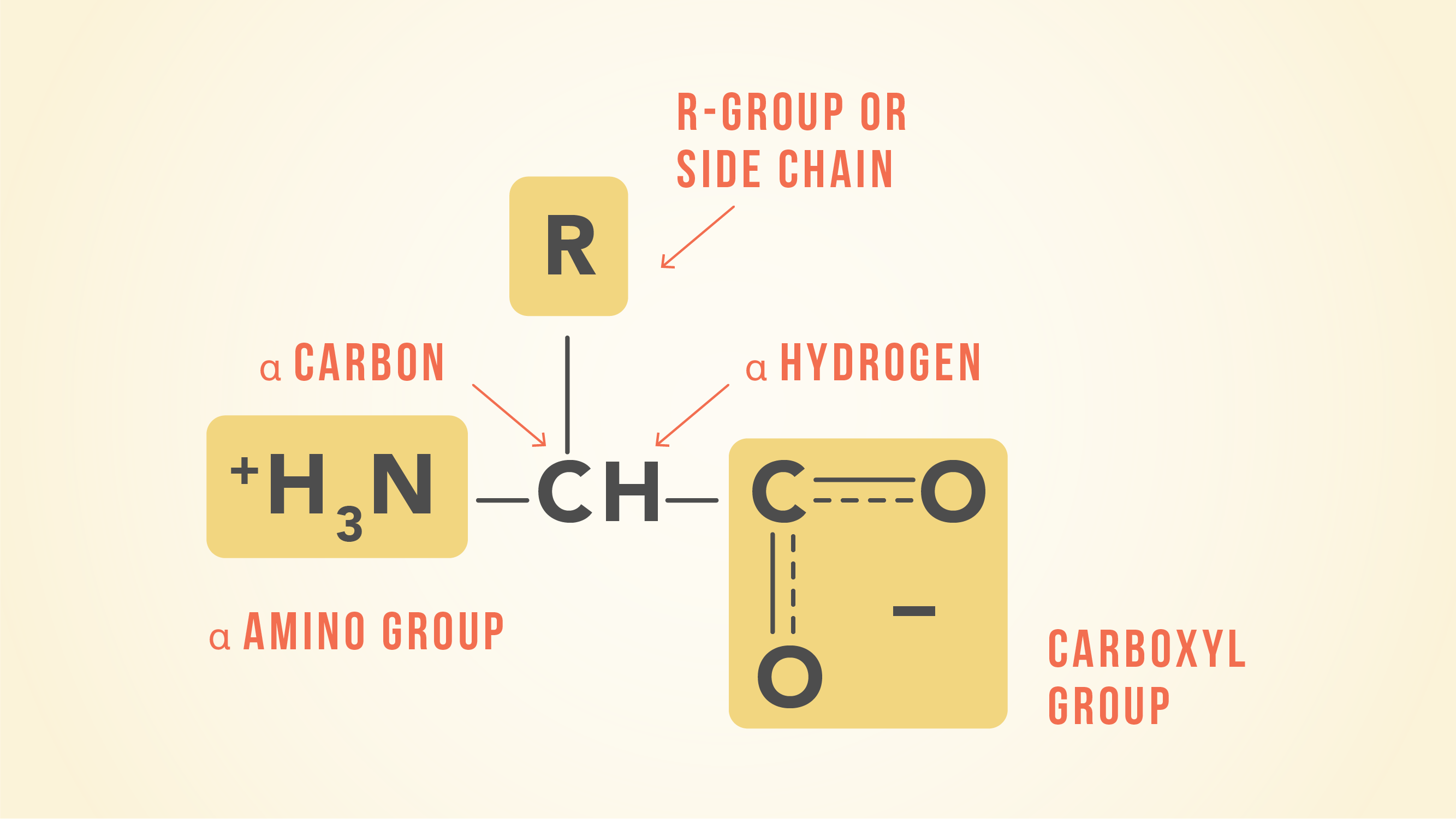
Would an amino acid with the given side chain be most likely to be found in the hydrophobic or hydrophilic region of a protein. The amino acids in proteins are α-amino acids which means the amino group is attached to the α-carbon of the carboxylic acid. On the third chain CH₂OH is present and the amino acid is serine.
Thus titration curves are helpful in the identification of amino acids as follows. The position of the amino acids in the chromatogram can be detected by spraying with ninhydrin which reacts with amino acids to yield highly coloured products purple. - Primary amine means the amino group contains at least two hydrogen with nitrogen.
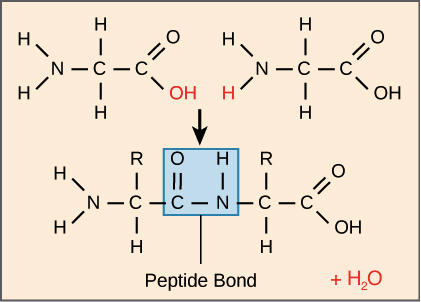
In this experiment we are finding out the titration curve of the amino acid Glycine. The standard structure contains both a carboxyl and an amine in the backbone. Common food sources for these amino acids include eggs soy protein and whitefish.
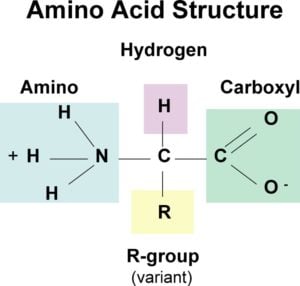
As the diagram below shows the absolute configuration of the amino acids can be shown with the H pointed to the rear the COOH groups pointing out to the left the R group to the right and the NH 3 group upwards. Fill in the incomplete structure below to give the structure of alanine. Charged carboxylate ion the α-amino group is a positively charged ammonium ion and the γ-carboxyl group is a neutral protonated acid.
COO- H3N CH - CH OH Serine. While neutral the zwitterion form of an amino acid will have a positive and a negative charge. There are three 3 Major types of Functional Groups based on the R group of the said amino acid.
These are amino acids or organic compounds that have no charge on the R group. There are six tests for the detection of functional groups in amino acids and proteins. 3 an example of a basic salt form.
To detect the presence of amino acid from a given unknown sample. Likewise if NaOH were added the resulting amino acid would be sodium glycinate see fig. Previous question Next question.
A mixture of unknown amino acids can be separated and identified by means of paper chromatography. Identify the outlined functional groups present in the amino acid and peptide compounds. Read this article to learn about the qualitative and quantitative tests for amino acids and proteins.
This is the zwitterion form of an amino acid. Unlike humans plants are capable of synthesizing all 20 amino acids. For more information about the α-carbon see Chapter 4 Carboxylic Acids Esters Section 42 Carboxylic Acids.
Hydrogen is also present in amino acids. Amino acids are building blocks of all proteins and are linked in series by peptide bond -CONH- to form the primary structure of a protein. Glycine is a diprotic amino acid which means that it has two dissociable Protons one on the α amino group and the other on the carboxyl group.
A titration curve is the plot of the pH versus the volume of titrant used. What elements are always present in amino acids. 1 Ninhydrin Test 2 Biuret Test 3 Xanthoproteic Test 4 Millons Test 5 Hopkins-Cole Test and 6 Nitroprusside Test.
- Secondary amine means the amino group contains one hydrogen on nitrogen. In the case of amino acids. And the amino group of a different amino acid Answer.
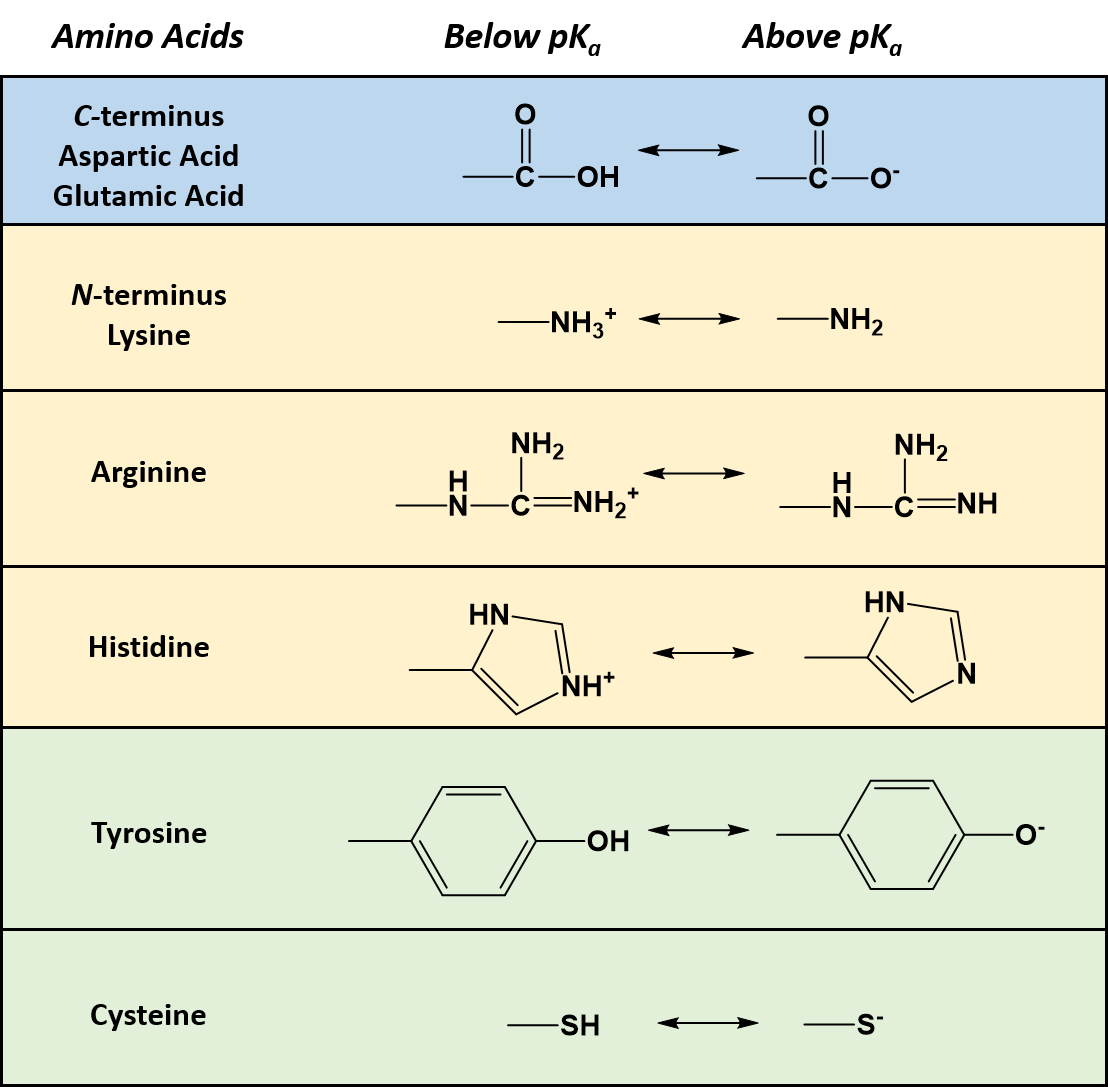
N- terminus -Glutamine- Isoleucine- Aspartate- Aspartate- Isoleucine- Threonine -Cysteine-Histidine C-terminus the overall charge of this peptide at pH 7 -2 Answer b- pI of this polypeptide 389 Note when pH pKa the side chain of the ionizable group would be protonated. Acidic and basic amino acids may have additional groups in their side chains. The carboxylic group is acidic and can be protonated at higher pHs to gain a negative charge.
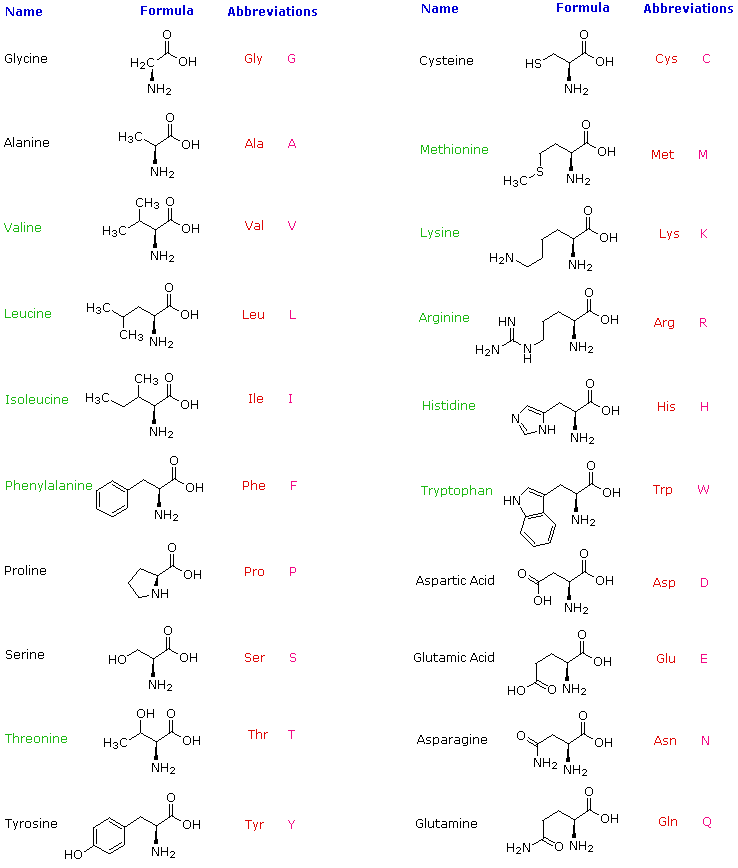
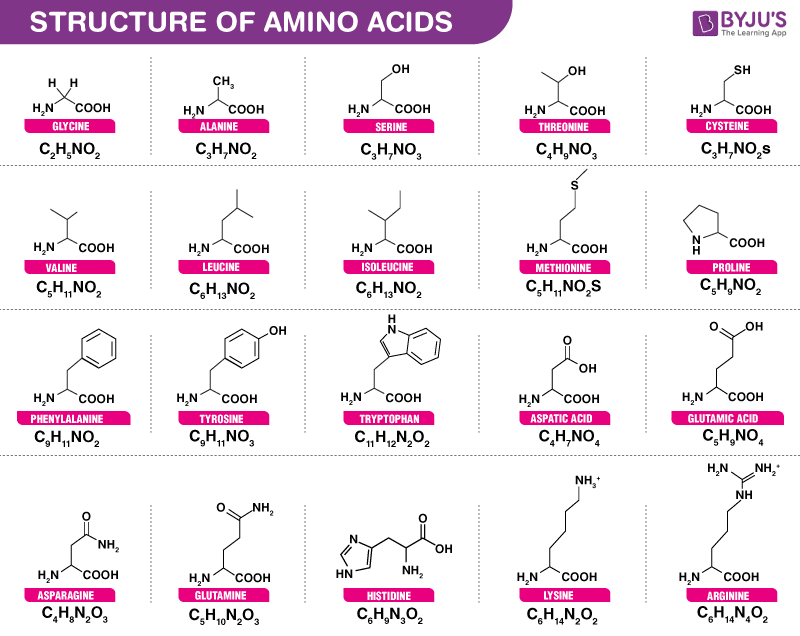
You can remember this with the anagram CORN. Write down the abbreviations both 1 letter and 3 letter for the amino acids given below A. The structure of Alanine is as follows.
The green NH₂ group is an amino group. The structure of Lysine is as follows. - By observing all the above structures we can easily identify the secondary amine which is present in proline.
So the correct option is i - Alanine ii Glycine iii Serine. The COOH is the carboxyl group. A 7 - CH -.
Chemistry questions and answers. Identify the R group of an achiral amino acid. 6 - CH 2 - CH - CH 3 A hydrophobic CH 3 B both Answer.
C00 Answer Bank CH3 carboxylic acid lonized amide alohal O TO- O а в е Answer carboxylic acid finired aldehyde ketone alcohol O amine onized H2N- TO- 0 CH H₃ C CH₃ buscas pracy policy. Amino acids contain an alpha-carboxylic acid group and an alpha-amino group. Identify the amino group present in the given amino acid.
Answer a- Amino Acid sequence of the given peptide is as follows. Identify the statement that is true about protonation and deprotonation of amino and carboxyl groups of amino acids. Complete the structure of the amino acid phenylalinine.
Free piperazinyl secondary amino groups are readily labeled with 11 Cmethyl iodide even in the absence of another base 1994EJNM131 1994NMB921 2010NMB205 2013JLCR120The synthesis of 11 C254 a dopamine transporter DAT radioligand is a typical example11 C254 was obtained in moderate overall yield by treating the piperazine 253 with. Nonpolar amino acids are hydrophobic which means they do not tend to move or combine with other aqueous compounds. For acidic amino acids the pI is given by ½pK1 pK2 and for basic amino acids its given by ½pK2 pK3.
The six tests are. Amino Acids with Nonpolar side chains. The amino group is basic which can lead to a positive charge when it is protonated at lower pHs.
Learn more about amino acid side chains link. Amino acids possess an amine group a carboxylic acid group and a varying side chain that differs between different amino acids. Which of the following amino acids has a positively charged R group.
Amines are organic compounds which is basic in nature so they dissolve in mineral acids like hydrochloric acid. - Coming to option D Lysine. Some elements that are always present in amino acids are carbon oxygen and nitrogen.
Fill in the incomplete structure below to give the structure of glutamate. 3 Due to the nature of amino acids a titration curve can be employed to identify an unknown amino acid. An amino acid contains a carboxyl group and an amine group and other groups depending on its structure.
COO- H3N CH - CH OH Serine. Similarly you may ask how can chromatography be used to identify. They are histidine isoleucine leucine lysine methionine phenylalanine threonine tryptophan and valine.
Hinsberg test a Solubility Test.

Amino Acids An Overview Sciencedirect Topics

Amino Acids Anatomy And Physiology I
25 2 Structure And Stereochemistry Of The Amino Acids Chemistry Libretexts
Amino Acids Introduction To Chemistry
Introduction To Proteins And Amino Acids Article Khan Academy
Essential Amino Acids Chart Abbreviations And Structure Technology Networks

Amino Acid Stereochemistry R And S Vs D And L Configuration Youtube

Amino Acids Introduction To Chemistry

Amino Acid Standard Amino Acids Britannica

Essential Amino Acids Chart Abbreviations And Structure Technology Networks

Essential Amino Acids Chart Abbreviations And Structure Technology Networks
Amino Acids The School Of Biomedical Sciences Wiki

Amino Acids Biology For Majors I
Amino Acids Definition Properties Structure Classification Functions
2 2 Structure Function Amino Acids Biology Libretexts

Chapter 2 Protein Structure Chemistry
Amino Acids Properties Functions Sources And Its Deficiency Disorders


Comments
Post a Comment